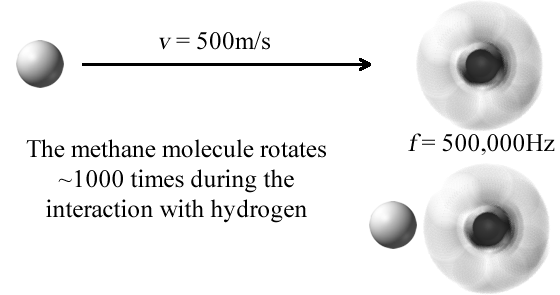Including orbital angular momentum
In our case of vibrational energy transfer, the rotations are so fast that we consider them to be adiabatic with respect to vibration frequency. The far right image of a methane molecule is how an incoming atomic collision percieves this "averaged" orientation.

For example, let's send in a carbon dioxide molecule directly at this rotating methane molecule.